Which of the Following Atoms Would Have the Greatest Velocity
Which of the following statements is true. 2 N 2 velocity 273 K.

Linearqualified Science 8 Density Calculations Worksheet Answers Science8 Check More At Htt Gas Laws Chemistry Teaching Chemistry Chemistry Classroom
Nitrogen has the greater value Xenon has the greater value Both gases have the same value.

. V 4613 ms. Which of the following atoms would have the greatest velocity if each atom had the same kinetic energy. Which objects have the greatest escape velocity.
Which of the following atoms would have the greatest velocity speed to diffuse if each atom had the same kinetic energy. V 3 831447 273 0028014. Argon has the biggest particles and is at the low temperature of 20C so it will be the gas with the highest speed.
Why is a gas easier to compress than a liquid or a solid. In Wikipedia look up Escape velocity scroll down to List of escape velocities and answer this question. Answer Fe N S Ti Cu.
Orbitals can hold two electrons provided they are spinning in the same direction This type of wave has certain allowable states or energies. It takes more force to move larger objects so applying the same amount of force to a smaller object would move it further and faster than applying the same force to a larger object. In which of the following materials is the velocity of light greatestA.
Oxygen is 16 times heavier than hydrogen on a per atom or per molecule comparison since both gases are diatomic in our everyday lives. This means the rms. The oxygen atom would have the greatest velocity due to its smaller atomic mass.
The greatest average velocity and the highest kinetic energy if Three 100 L flasks at 25oC and 725 torr contain the gases CH4 flask A CO2 flask B and C2H6 flask C. V 3RT M. Daltons idea that atoms cannot be divided into smaller parts was disproved by the discovery of _____ electrons 2.
V 3 831447 273 00319988. States of Matter. A ball-and-stick model of ethanol is made from the following components.
Hydrogen Which does the ideal gas law allow a scientist to calculate that the other laws do not. Score 1BubblesPoints 8038 User. C one silicon atom for every two oxygen atoms.
Asked by Pszpila Last updated. 493 4613 10687 N 2 moves about 107 times as. D 5 means d subshell has 5 unpaired electrons which is greatest among d-subshells.
It takes more force to move larger objects so. The velocity of light is greatest in air. Gas for which the molecules or atoms have the greatest average velocity.
Rich Text Editor your_expand_ans_box. V 493 ms. Question 17 Question 17 1.
Moons Question 2 1 point. At low temperatures and pressures how does the volume of a real gas compare with the. Which of the following atoms has the greatest number of unpaired electrons in its ground state.
5 points Question 18 Question 18 1. The oxygen atom would have the greatest velocity due to its smaller atomic mass. Here are our choices.
The description of the structure of the atom is called _____ atomic. Answer neutron nucleus proton electron electron or the proton. The velocity of a sound wave is the greatest in C Air Sound has a velocity of about 033 km per second 02 mile per second in air 15 km per second in.
An atom has 3 protons 4 neutrons and 3 electrons. 3 N 2 moves faster. B two nitrogen atoms for every four oxygen atoms.
The molecules of which of the following gas have highest speed. Ernest Rutherfords model of the atom did not specifically include the ____. 1 O 2 velocity 273 K.
A molecule of ethanol has two carbon atoms six hydrogen atoms and one oxygen atom. A one calcium atom for every two iodide atoms. Cr has d 5 configuration in outermost shell.
-One red ball -Two black balls -Six white balls -Eight sticks What do. RMS speed is inversely proportional to the square root of mass molecular or molar. Hydrogen gas has the lowest mass out of ammonia bromine hydrogen and chlorine so it would have the highest velocity for a given kinetic energy.
A particle A moving with a certain velocity has a de Broglie wavelength of 1 A If the particle B has mass 25 of that of A and velocity 75 of that of A then the de-Broglie wavelength of B will be approximate. Write the chemical formulas for the compounds containing each of the following. Helium has both the smallest particles and is at the high temperature of 100C so it will be the gas with the highest speed.
The partial pressure of N is 101 kPa. Which of the following atoms would have the greatest velocity if each atom had the same kinetic energy. Which one of the following statements about orbitals is incorrect.
The molecules of which of the following. Solve Study Textbooks Guides. When white light is incident on a prism which one of the resulting color components will have the lowest index of refractionA.
Kinetic Energy and Molecular Speeds. Gas that would have a higher rate of effusion through a small hole opened in the flask.

Bohr Diagram Of Sodium Atom Model Atom Diagram Atom Model Project
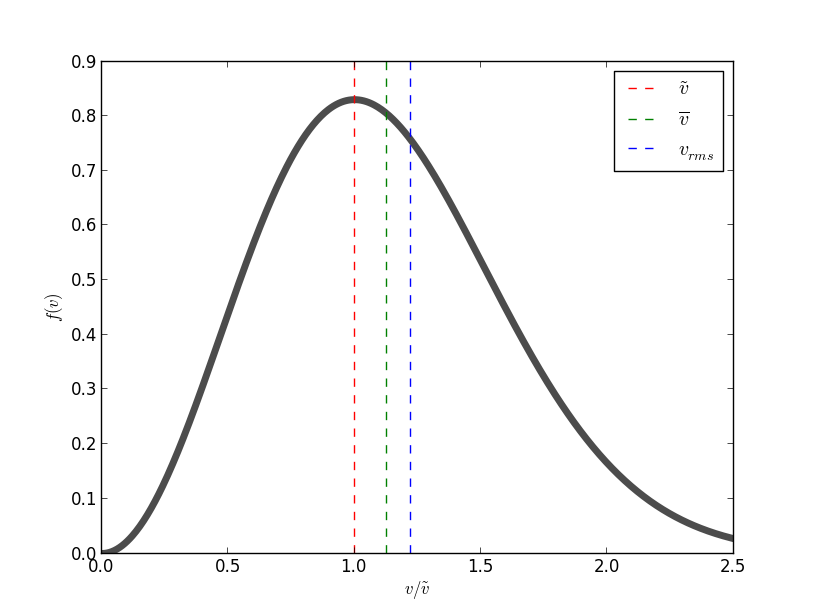
What Are The Most Probable Velocity And The Average Velocity For A System That Follows The Maxwell Boltzmann Distribution Socratic

Bohr Model Hydrogen Atom Postulates Energy Levels Bohr Model Atom Model Hydrogen Atom

History Of The Atom Atomic Theory Atom Subject And Predicate Worksheets

Sublevel Easy Science Study Skills Electron Configuration Energy Level

Freely Electrons Electron Drift Velocity Drift Velocity Electrons Electrical Engineering Projects
13 3 Stream Erosion And Deposition Physical Geology

Tight Binding Model For P Electrons On Honeycomb Lattice Electrons Microscopic Images Real Pictures

Atomic Structure Atom Model Proton Neutron Electron O Levels
No comments for "Which of the Following Atoms Would Have the Greatest Velocity"
Post a Comment